
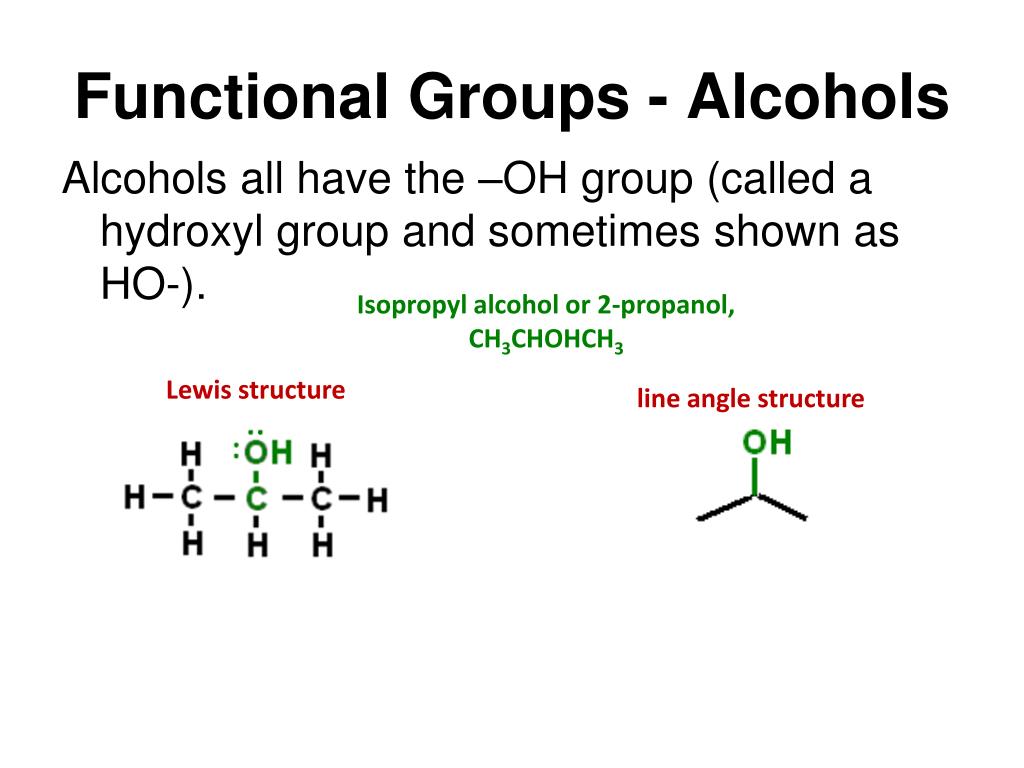
Again, the carbon chain is numbered to give the OH group the lowest number, no matter how large the other numbers are. For larger alcohol molecules, we use a number to indicate the position of the OH group on the longest carbon chain, similar to the number needed for alkenes and alkynes. Thus CH 3OH is methanol and CH 3CH 2OH is ethanol. The formal system of naming uses the name of the hydrocarbon containing the OH group and having the correct number of C atoms, dropping the final – e of the name and appending the suffix – ol. So CH 3OH is methyl alcohol, CH 3CH 2OH is ethyl alcohol, and CH 3CH 2CH 2OH is propyl alcohol.Īs with alkyl halides, though, this system is limited (although for smaller alcohols, it is very common in everyday usage). The common system is similar to that of alkyl halides: name the alkyl group attached to the OH group, ending with the suffix – yl, and add the word alcohol as a second word. Like alkyl halides, alcohols have a common naming system and a more formal system. It is not the hydroxide ion in organic chemistry, rather than being present as a negatively charged species, it is a covalently bonded functional group. The correct name for this molecule is 2,3-dichloro-3-methylpentane.Īnother simple functional group is the covalently bonded OH group. There are two chlorine substituents located on the second and third C atoms, with a one-carbon methyl group on the third C atom as well. The longest carbon chain has five C atoms, so the molecule is a pentane. Numerical prefixes are ignored when determining the alphabetical ordering of substituent groups. If alkyl groups are present, the substituents are listed alphabetically. For example, this molecule is 2-bromobutane.Īnd this molecule is 2,3-dichloropentane: If there is more than one of a certain halogen, we use numerical prefixes to indicate the number of each kind, just as with alkyl groups.
ALCOHOL FUNCTIONAL GROUP PLUS
The name of the halogen as a substituent comes from the stem of the element’s name plus the ending – o, so the substituent names are fluoro-, chloro-, bromo-, and iodo. The systematic way of naming alkyl halides is to name the halogen as a substituent, just like an alkyl group, and use numbers to indicate the position of the halogen atom on the main chain. However, this system is not ideal for more complicated alkyl halides. So CH 3Cl has the common name of methyl chloride, while CH 3CH 2Br is ethyl bromide and CH 3CH 2CH 2I is propyl iodide. We have already seen some examples of alkyl halides when the addition of halogens across double and triple bonds was introduced in the section “Branched Hydrocarbons” the products of these reactions were alkyl halides.Ī simple alkyl halide can be named like an ionic salt, first by stating the name of the parent alkane as a substituent group (with the – yl suffix) and then the name of the halogen as if it were the anion. Organic compounds that contain a halogen atom are called alkyl halides. The presence of a halogen atom (F, Cl, Br, or I X is used to represent any halogen atom) is one of the simplest functional groups. They undergo certain characteristic chemical reactions - for example, the addition of a halogen across the multiple bond. We have already seen two functional groups: the C–C double bond and the C–C triple bond.




 0 kommentar(er)
0 kommentar(er)
